Puberty Blockers Fast-Track Children Toward Full Gender Transition
How to best care for the skyrocketing numbers of trans-identifying youth is one of the most urgent problems facing clinicians today.
Reality’s Last Stand is a reader-supported publication. Most articles are free, so if you would pay to read this article, please consider becoming a paying subscriber anyway or making a one-time or recurring donation to show your support. I’d rather a million people read RLS for free than have it be accessible to only a small group of paying subscribers, but that means I rely fully on the generosity of my readers for support. Thank you!
This article was originally published on the Society for Evidence-based Gender Medicine’s website on February 2, 2023.
A recent study published in The Journal of Sexual Medicine reported demographic and treatment trends among gender-dysphoric youth seeking evaluation and/or treatment at the Netherlands’ largest pediatric gender clinic in Amsterdam (VUmc) between 1997 and 2018. The study seemingly supports the emerging narrative that “gender-affirming” care for youth has been thoroughly tested over 2 decades; that the long-term trajectories of gender-transitioned youth are both well-understood and positive, as evidenced by virtually no detransition; and that in fact, many “transgender adolescents” do not want any medical interventions—but for those who desire them, puberty blockers, cross-sex hormones and surgery should be widely available, as long as the adolescents are “comprehensively assessed.”
However, a closer examination reveals that these assertions are not supported by the data presented. Below, we briefly explain the design of the study and highlight the study’s main findings. Next, we analyze several key assertions made by the study authors that are not supported by the data. Finally, we discuss the implications of continuing to scale “gender-affirming” medical interventions to the rapidly growing numbers of youth seeking gender reassignment absent reliable research of long-term outcomes.
Brief Study Description
The study reported demographic and treatment trends in 1,766 patients who were under age 18 when they first presented to Amsterdam’s VUmc pediatric gender identity clinic for evaluation or treatment with puberty blockers. This retrospective study reported the age and sex of referrals to the youth gender clinic between 1997 and 2018 and the rate of uptake of each intervention phase of “gender-affirming” care: puberty blockers, cross-sex-hormones, and surgery. It also reported the conversion rate from puberty blockers to cross-sex hormones.
All trends were analyzed for two distinct groups of patients: those who sought their first evaluations before age 10 and those who presented to the clinic at age 10 or older (defined as “early” vs “late-presenting” groups).
The Study’s Key Findings
Below we enumerate the most relevant points that are well-supported by the study data:
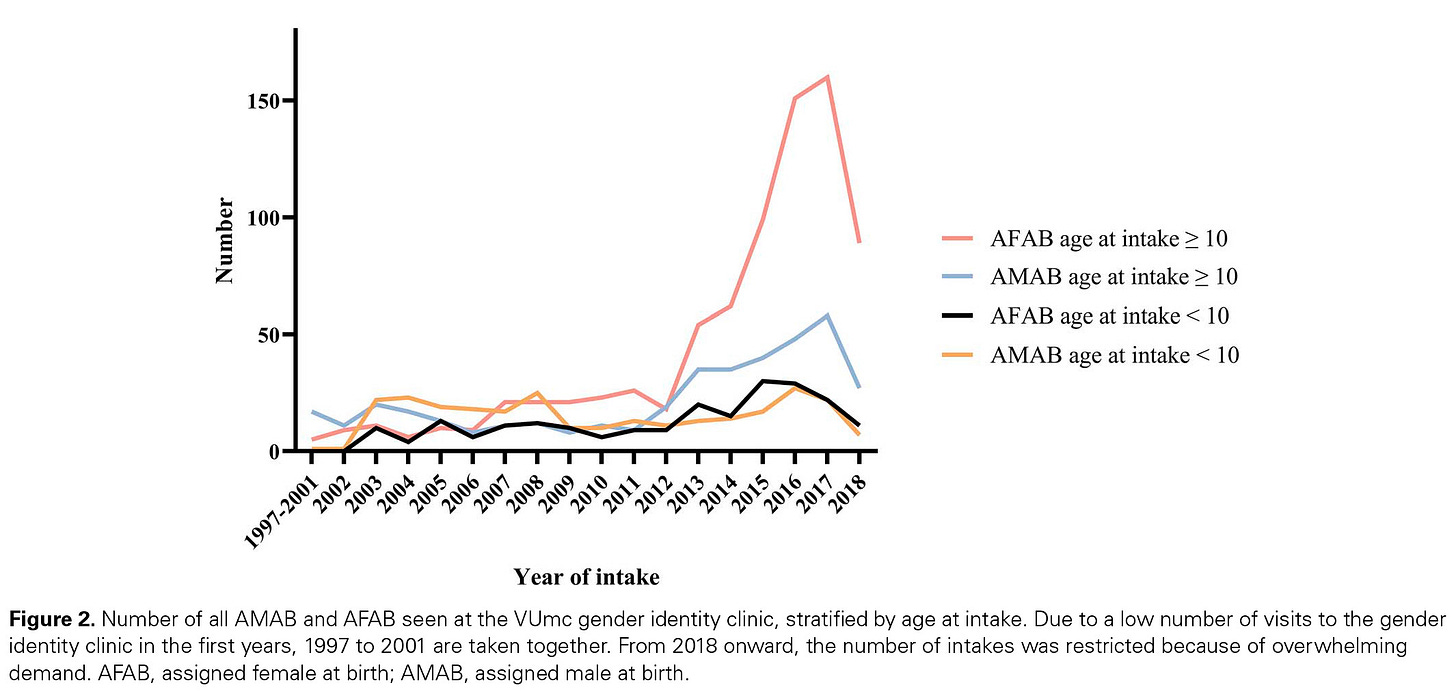
There was a sharp increase in referrals to the Amsterdam clinic, driven by late-presenting females.
The study confirms the epidemiologic trend reported throughout the Western world: a surge in late-presenting cases of gender dysphoric youth, primarily driven by adolescent females. In the Netherlands, this trend began a bit earlier than in the rest of the Western world, circa 2012.
Note: as the footnote to the graph indicates, the drop in 2018 observed in Figure 2 (above) does not represent a drop in demand, but rather, redirection of new cases to other clinics in order to keep up with growing demand for services.
The majority of late-presenting cases were female, while the majority of early-presenting cases were male.
The study reported that 67% of late-presenting cases were female (795 out of 1194), compared to only 45% of the early-presenting cases (217 out of 487). (These numbers can be found in Figure 1 in the paper.)
The majority of late-presenting cases underwent medical transition.
The study reported that 77% of the late-presenting “potentially eligible” females started puberty blockers at the Amsterdam clinic (this number was 53% for the late-presenting males). More than 90% of both late-presenting “potentially eligible” males and females started cross-sex hormones at the clinic.
A significant number of early-presenting cases of both sexes did not transition upon puberty.
The data indicate that only 36% of the “potentially eligible” early-presenting males and 53% of the “potentially eligible” early-presenting females pursued medical transition once they became eligible during puberty, while 64% and 47% respectively did not (see Table 2 in the study).
The authors also note that “a substantial proportion of children first visiting before age 10 years did not meet criteria for a GD [gender dysphoria] diagnosis.” The extent to which this may be a reflection of the well-established phenomenon of high rates of desistance among those with early-onset gender dysphoria is unclear, as the authors did not specify whether these youth had ever met the criteria for a GD diagnosis.
Females were much more likely than males to initiate medical transition.
More than three-quarters (77%) of late-presenting “potentially eligible” females started puberty blockers at the Amsterdam clinic, compared to slightly more than half (53%) of the late-presenting males. Similar differences between the male and female subjects were observed for the early-presenting cases (53% of females and 36% of males pursued medical transition eventually).
The authors note this marked gap between females and males starting puberty blockers: “The difference between AMAB [males] and AFAB [females] in the relative number of people starting GnRHa [puberty blockers] is remarkable.”
There was a high rate of progression from puberty blockers to cross-sex hormones.
The study found a high rate of conversion from puberty blockers to cross-sex hormones—93%-98%. The authors concede that puberty blockers may not serve as a diagnostic tool as previously thought, but rather represent the first step in medical gender transition. The authors also hypothesize that it might be possible that “starting GnRHa in itself makes adolescents more likely to continue medical transition.”
Surgeries continue to be a core part of the treatment path, but fewer patients are opting to remove gonads (ovaries and testes) since sterilization requirements were lifted in 2014.
Following changes in Dutch laws in 2014, which allowed for legal recognition of “sex change” without undergoing sterilization, the number of young people who sought surgery to remove gonads dropped but remained significant. Since 2014, 53% of “potentially eligible” biological males and 38% of “potentially eligible” biological females underwent gonadectomy. A significant proportion of “potentially eligible” females underwent mastectomy (79%). However, mastectomy rates were reported in the aggregate across the entire 20-year span, and the definition of “potential eligibility” for surgery may have inadvertently excluded some youth under 18 who underwent mastectomy in the later years of the study (see point 6, “The reliance on ‘potential’ eligibility” below).
Potentially Misleading Claims
The authors make several claims which, while technically accurate, may inadvertently be misleading. Below we highlight some of these instances and demonstrate the importance and value of reading this paper carefully, rather than accepting its statements at face value.
1. The study claims to represent “the first 20 years of the Dutch Protocol,” but fails to acknowledge that the Dutch Protocol’s strict eligibility criteria were not consistently followed during the study period.
The Dutch Protocol has become internationally synonymous with the careful and cautious approach the Dutch clinicians devised and documented in 1997, 2006, 2008 and 2012. It required an early childhood onset of gender dysphoria, increase of gender dysphoria after pubertal changes, absence of significant psychiatric comorbidity, and demonstrated knowledge and understanding of the consequences of medical transition. Treatment with puberty blockers could only be initiated at the minimum age of 12. Interventions with clearly irreversible effects—cross-sex hormones and surgery—were not available until ages 16 and 18, respectively. All youth were provided with psychotherapy throughout. The Dutch Protocol specified that youth with “nonbinary” presentations were ineligible for medical interventions, and instead should be treated with psychotherapy.
It is therefore surprising that the authors of the study “redefined” the Dutch Protocol as merely a “diagnostic procedure and combined treatment of GnRHa [puberty blockers] and subsequent GAH [cross-sex hormones]” (p.2)—without ever mentioning the Protocol’s strict eligibility requirements, which have long been juxtaposed to the much more permissive practice of “gender affirmation” that began to proliferate in the West after the publication of the Dutch Protocol’s outcome data in 2014. (The origins of the Dutch Protocol, and the outcomes of the 2014 study that launched the practice of youth gender transitions worldwide, have recently been critically evaluated).
It is even more surprising that the authors refer to the “Dutch Protocol” and Endocrine Society guidelines by Hembree et al. interchangeably (p. 2). The Endocrine Society guidelines were only issued in 2017, while the study reports on cases assessed and treated between 2006 and 2018. In addition, there are clear differences between the Endocrine Society guidelines and the criteria outlined in the Dutch Protocol in terms of eligibility requirements. Unlike the Dutch Protocol, the Endocrine Society guidelines do not require a childhood onset of gender dysphoria, nor do they set the minimum age for puberty blockers at 12. Instead, the Endocrine Society permits the use of puberty blockers at Tanner Stage 2 of puberty, which can occur in girls as young as ages 8 or 9.
The authors make several contradictory claims about the fidelity of adherence to the Dutch Protocol’s requirements throughout the 20-year period. They state that the clinic “followed 1 diagnostic and treatment protocol” over 20 years, but also volunteer that “the protocol was adapted” and that “practice has evolved since the start of the Dutch Protocol.” It is unclear when the Amsterdam clinic began to deviate from the strict Dutch Protocol, which was the basis for the seminal 2014 Dutch study. It is clear, however, that as of 2018, the official Somatic (medical) treatment guidelines from the Netherlands no longer adhered to the Dutch Protocol.
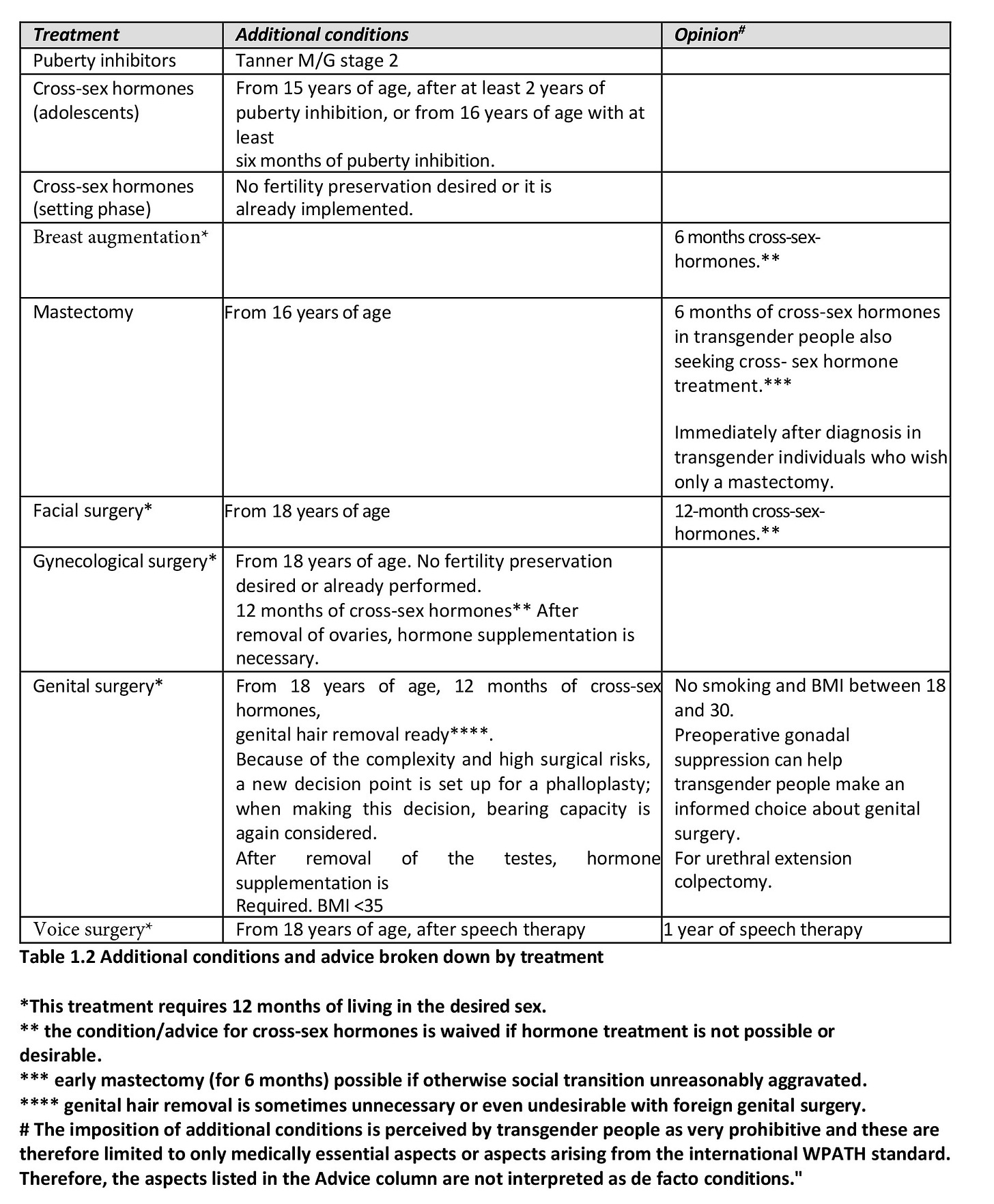
According to the 2018 Somatic (medical) treatment guidelines, eligibility for medical transition no longer requires a history of early onset of gender dysphoria—instead, adolescent-onset cases could be medically transitioned. Table 1.2 of the document (auto-translated and reproduced below) indicates that the minimum age at which puberty blockers may be initiated was lowered from age 12 to Tanner stage 2 (which can occur at ages considerably younger than 12), while the eligibility for cross-sex hormones was lowered to age 15 (and the text suggests even younger ages may be considered). The age of eligibility for mastectomy was lowered from 18 to 16. Further, it is unclear whether the DSM-5 diagnosis of “Gender Dysphoria” (with its key element of gender-related distress) is still required to initiate treatment, since the document only refers to the ICD-11 diagnosis of “Gender Incongruence” which has no distress criterion.
While it is expected that clinical practice evolves, it is not appropriate to refer to the “Dutch Protocol” when describing a practice that uses the Protocol’s highly invasive medical and surgical interventions but ignores its clearly articulated eligibility requirements.
The authors state that the goal of the study was to “review how practice has evolved since the start of the Dutch Protocol and to evaluate the treatment trajectories of people who were treated accordingly.” Evaluating the trajectories of youth treated under far less restrictive criteria is critical. However, this question is not answered by the study, since data were not evaluated separately for those treated under the Dutch Protocol and those who were treated under the newer “evolved practice” criteria.
2. The “unprecedented" 20-year time span of the study, listed as the study’s key strength in the abstract, masks a much shorter 4.6-year median patient-level follow up.
While it is technically true that the study data span over 20 years, what this statement fails to address is that in terms of patient-level treatment trajectories, the median follow up is only 4.6 years from the first intake appointment. For 25% of the sample, follow up is less than 3 years from the first intake appointment.
The intake appointment was followed by “comprehensive assessments,” that the authors say are critical, and which presumably took several months to complete. Further, as the study data show, puberty blockers were administered for another 1-2 years. Therefore, a substantial number of youth presenting to the clinic in the later years would have only just started cross-sex hormones around 2017-2018, just as data collection was drawing to a close. Even fewer of them would have undergone surgery by the end of data collection.
To properly evaluate outcomes, follow up consisting of months to a few years after starting medical treatment is insufficient. Prior research suggests that health problems in general, as well as psychological problems and regret often do not peak until a decade later. Recent research from the US, which has been following less restrictive “affirmative care” protocols outlined by the Endocrine Society, reveals that 30% discontinued hormones just 4 years after treatment initiation—a surprisingly high rate, considering that these treatments are intended to be used life-long in order to maintain a feminized or masculinized appearance.
Since the bulk of cases included in this “20-year” study occurred in the last few years of the study, currently available data simply do not extend out far enough to provide reliable information about eventual health outcomes.
3. The study claims that among youth who initiated medical transition, “detransition was very rare”—but the authors never evaluated detransition rates.
The study conclusion states: “detransition was very rare.” This finding has also been elevated into the study abstract, which says: “the risk of retransitioning was very low.” Aside from creating confusion regarding terminology (“detransition” and “retransition” which the authors use interchangeably), these statements may lead readers to the incorrect conclusion that most youth who had embarked on medical treatment stayed on it long term. However, the study never attempted to evaluate the rate of detransition. The only “detransition” rate assessed was detransitioning during the puberty blocker phase. The study did not evaluate detransition rates for youth on cross-sex hormones or those who had undergone surgery.
The authors did find a low rate of detransition during the puberty blocker phase: 93%-98% of youth who started puberty blockers did not discontinue them, and instead proceeded to start cross-sex hormones. This finding led the authors to appropriately conclude that puberty blockers may not serve as a diagnostic tool, but rather represent the first step in medical gender transition—and they even suggest that treatment with puberty blockers may contribute to the high incidence of subsequent treatment with cross-sex hormones.
When it comes to the question of detransition, most readers of the paper want to know: What percent of youth who start medical transition eventually detransition? What is the average time to detransition? Do some change their mind again and retransition, and what is the timeframe for this decision? Unfortunately, this particular study cannot answer these important questions because the authors never analyzed treatment discontinuation rates for youth treated with cross-sex-hormones or surgery.
4. The claim that “a substantial number of adolescents did not start medical treatment” problematically conflates “medical treatment” with “puberty blockers” and masks the fact that the vast majority of “late-presenting” females (which is now the predominant presentation) DID transition.
The statement in the abstract, “a substantial number of adolescents did not start medical treatment,” while technically accurate, may lead readers to the erroneous conclusion that a substantial number of trans-identified adolescents seen at the Amsterdam clinic did not receive any medical interventions. This, in turn, may signal that the growing international concern with overmedicalizing gender-dysphoric youth is little more than a “moral panic.” Yet, a close reading of the study shows that 77% of “potentially eligible” late-presenting adolescent females (which is now the predominant presentation) DID in fact transition. And as the analysis below suggests, even this number may be a significant underestimate.
The claim in the abstract can be traced to the sentence in the body of the paper, “63% of all 1401 adolescents potentially eligible for GnRHa [puberty blockers] at the end of data collection had started GnRHa” (p. 4). This sentence does indicate that 37% of those assessed at the clinic—a substantial proportion—were not treated with puberty blockers. What it does not mean, however, is that 37% of gender-dysphoric adolescents did not undergo medical transition:
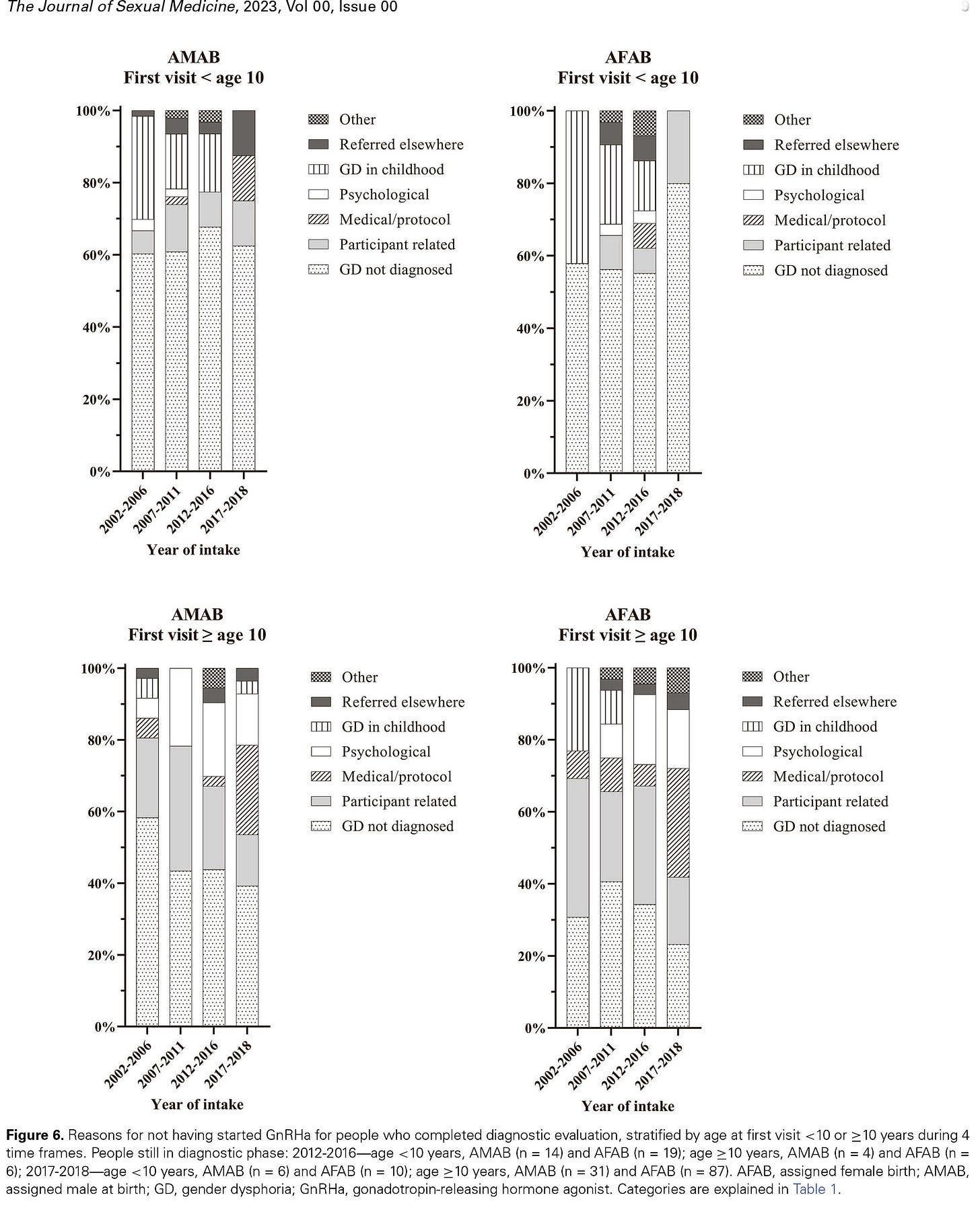
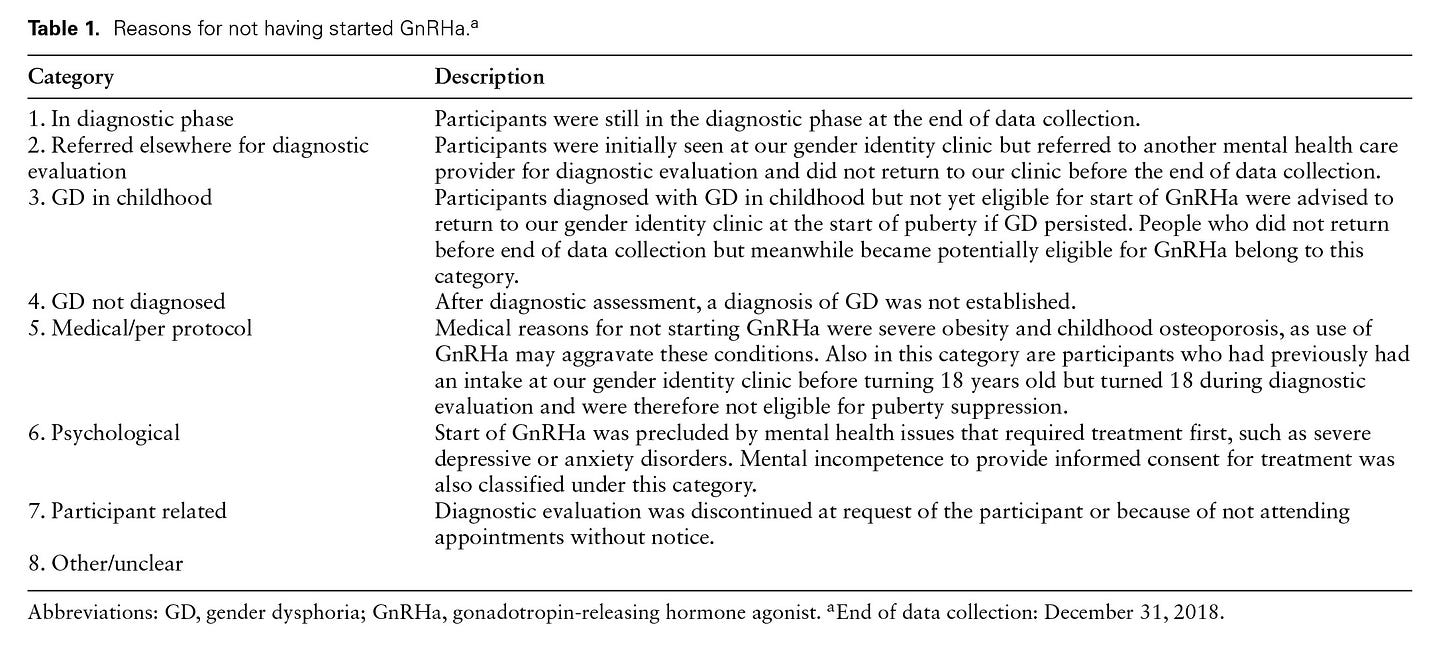
The study’s definition of “did not start medical treatment” is not equivalent to “did not undergo medical gender transition.” The study problematically defines “starting medical treatment” as starting puberty blockers. However, one could start medical treatment at the cross-sex hormone treatment phase. For example, a significant number of cases listed as “not starting treatment” did so due to “medical/protocol” reasons (see Figure 6). As Table 1 below indicates, “medical/protocol” reasons subsumed youth who turned 18 during the evaluation and became eligible to bypass puberty blockers and start directly with cross-sex hormones. Thus, using “starting medical treatment” interchangeably with “starting puberty blockers” is misleading, as it results in what appears to be a relatively low number of those who “started medical treatment.”
The denominator for the calculation was inflated by inclusion of “potentially eligible” subjects who were not actually eligible. The calculation of the percent “starting medical treatment” is based on the “potentially eligible” cases. However, the definition of “potential eligibility” for puberty blockers used in the study has little to do with actual eligibility. “Potential eligibility” was defined simply as “minimum age of 12 [presumably as of the end of 2018] and at least 1 year after the first visit.” “Potentially eligible” cases apparently include youth not diagnosed with gender dysphoria upon assessment (“GD not diagnosed”); youth who may have desisted (“Participant related”); and the “watchful waiting” group of children whose gender dysphoria resolved (“GD in childhood”), according to Figure 6 below. The inclusion of “potentially eligible” but not actually eligible cases in the denominator led to a lower “percentage treated” number than would have been reported had the calculation been based on the actually eligible cases.
The calculation fails to account for all those treated with puberty blockers. There are several scenarios in which youth who likely received puberty blockers were not counted as such. First, youth who were “referred elsewhere” (see Figure 6 below) may have started puberty blockers at other clinics. These youth are in the denominator, but not in the numerator. Second, youth who were referred in the last few years of the study may not have yet become eligible for puberty blockade but may have pursued these interventions in the months following the end of data collection. The authors do note this possibility. Third, because of the unusual definition of “potential eligibility,” some youth who were actually treated with puberty blockers during the study period were likely excluded from the calculation entirely. For example, since the calculation required that the subject be at least 12 years of age at the end of 2018 (a key element of “potential eligibility”), a 10-year-old child treated with puberty blockers in 2017, who would not have yet turned 12 as of the end of 2018, would have been excluded. This phenomenon likely disproportionately impacts those treated in the last few years of the study–which is when the Amsterdam clinic likely began to deviate from the Dutch criteria of minimum age of 12 and began to treat youth according to Tanner stage.
A review of Figure 6 and Table 1 below makes it apparent that the 37% rate of “non-treatment” is difficult to interpret even when it comes to assessing the rate of starting puberty blockers. This number is even less reliable if one wishes to know, more generally, the percentage of initial referrals who were found eligible for medical treatment, as well as the percentage of those who subsequently medically transitioned.
The relatively low percentage of those “medically treated” reported by the study appears to bolster the assertion that many transgender-identifying youth simply self-express their unique identities, and that many do not receive medical interventions. This assertion was recently made for the first time by the American Academy of Pediatrics, to assuage mounting concern that rapidly growing numbers of youth are being subjected to hormones and surgeries with irreversible consequences.
Therefore, it would be highly informative to know: What percentage of gender-dysphoric adolescents presenting to the Amsterdam clinic in recent years were diagnosed with gender dysphoria? How many were “cleared” to start medical transition? How many actually initiated medical transition in the months and years that followed? How have these trends changed over time?
Unfortunately, the data presented in this study make it impossible to answer these important questions.
5. The claim in the abstract that the results “provide ongoing support for medical interventions in comprehensively assessed gender diverse adolescents” is inaccurate.
The assertion that the study results support the continuing treatment of youth with puberty blockers, cross-sex hormones, and surgery is highly misleading. Such a conclusion is unjustified for several reasons. Besides failing to estimate the rates of desistance and/or regret after starting cross-sex hormones over a sufficient timeframe, the study also fails to examine the physical and mental health outcomes of the adolescents.
To answer these questions, all relevant outcomes must be analyzed over a sufficient timeframe. Further, treatment with puberty blockers and cross-sex hormones must be compared to alternatives (such as the choice not to treat medically, or to use noninvasive approaches such as psychotherapy) and show clear evidence that the benefits of medical and surgical interventions outweigh the risks.
6. The study’s disclosure of limitations, particularly in the abstract, is inadequate.
Although the authors mention several critically important limitations in the body of the study, the only one elevated into the abstract is the study’s “retrospective design.” As many busy clinicians only read abstracts, this would lead them to believe that the study is effectively free from significant limitations. We describe some of the most important ones below.
The “20-year sample” is heavily skewed toward recent cases, the ultimate trajectories of which are essentially unknown.
A significant number of cases came from the “explosion” in referrals in the last few years of the study, especially 2017-2018. The authors do mention that “caution needs to be taken when interpreting results from the most recent years” and that calculated proportions of youth undergoing treatment may be an underestimation. However, this key limitation is not acknowledged in the abstract, which instead states that current data “provide support for ongoing medical interventions in comprehensively assessed gender diverse adolescents.”
The reliance on “potential eligibility” rather than actual eligibility for medical interventions resulted in highly uncertain estimates that likely underestimated treatment uptake rates among those actually eligible for treatment.
As discussed earlier, reliance on “potential eligibility” when reporting on treatment trends is a double-barreled problem in this study. On the one hand, this definition includes many “false positive” cases, such as those not diagnosed with gender dysphoria. On the other hand, it may exclude some “false negatives,” in which treatment deviated from the strict Dutch Protocol eligibility requirements, especially in the later years.
Based on the definition of “potential eligibility” for puberty blockers, 10-year-olds starting puberty blockade in 2017 (or 9-year-olds starting in 2016) likely would have been excluded from the trend analyses, as they would not have turned 12 by the time data collection ended in 2018 (the minimum age of 12 by the end of 2018 was a necessary condition for inclusion). Likewise, 14-year-olds treated with cross-sex hormones in 2017 would likely have been excluded as well (although the study's definition of “potential eligibility” for cross-sex-hormones is less clear). And according to the definition of “potential eligibility” for surgery, those receiving mastectomies at age 16 in 2017 or 2018 were also likely excluded, since the requirement of the minimum age of 18 was applied to the surgical trends analysis.
Using the Dutch Protocol’s minimum age criteria for reporting, while lowering the minimum ages in actual practice, introduced a serious risk of bias. It likely systematically excluded those treated at younger ages than specified by the original Protocol, especially in the last 1-2 years of the study.
The utility of the statistics presented as a 20-year average is compromised by a skewed sample.
Many of the important statistics—and all the statistics in Tables 2 (treatments with puberty blockers and hormones) and 3 (treatments with surgery) are reported in the aggregate over 20 years. It is well recognized that adolescent referrals surged in recent years and the presentation of gender dysphoria markedly changed. It is also apparent that the Dutch Protocol criteria were not closely followed by the Amsterdam clinic in the last several years. Thus, these data are of little utility for readers interested in current treatment patterns and patient treatment trajectories.
One of the study’s key findings is that younger gender-dysphoric children are less likely to get medical treatment once they reach adolescence. To the extent that the relatively low rates of treatment initiation in the “early-presenting group” are in part due to high rates of childhood desistance, the study unfortunately conflates the earlier time period when pre-pubertal social transition was explicitly discouraged by the Dutch Protocol, and the more recent years when the practice was much more common. Early social transition may have led to higher rates of persistence in the final years of the study, but this question cannot be answered because these data were only reported in the aggregate across 20 years.
The apparent attempts to differentiate youth with childhood vs adolescent-emergence of transgender identity relies on a crude and hard-to-interpret “presentation before vs after 10 years of age.”
The question of how to best treat youth with post-pubertal emergence of transgender identity is one of the most important questions since this has become the predominant presentation, and these youth were excluded from the Dutch Protocol eligibility in the past. A recent grant to the Amsterdam research team will be used to study the risk/benefit ratio of medically transitioning this novel patient cohort.
The lead researcher of the Dutch Protocol and a co-author of this study, Annelou de Vries, has acknowledged that youth presenting with post-pubertal emergence of transgender identity may benefit from psychological interventions rather than hormones and surgery. While this hypothesis is not addressed in the current study, the study does appear to attempt to approximate the novel cohort by presenting the data in two groups split by age of referral (younger and older than age 10 at intake). Specifically, the authors say:
To study whether there is a difference in the proportion of people starting GnRHa between those who were prepubertal and pubertal at their first visit, we compared those who had their first visit at age <10 and >=10.
However, the cut-off based on the age of 10 is too blunt a measure, given the wide range of age of the onset of puberty, and the significant differences in pubertal timing in girls and boys. It is also unclear why age 10 was chosen as the cut-off when another recent paper reporting on effectively the same cohort presenting to the Amsterdam clinic defined early vs late presenters based on whether they were age 14, rather than age 10.
Using consistent age cut-offs to distinguish between the “early” vs “late-presenting” cases would help eliminate key inconsistencies between recent studies from the Amsterdam gender clinic. For example, the most recent paper in question reported that the “early-presenting” cases were predominantly male, while the “late-presenting” cases were predominantly female, and found that the “late-presenting” cases were more likely to pursue medical interventions than the “early-presenting” cases. However, another recent paper studying the same patient cohort found just the opposite: that the “late-presenting” cases were less likely to pursue medical interventions, and that both the “early” and “late-presenting” cases were predominantly female.
However, in order to differentiate pre-pubertal vs pubertal onset of gender dysphoria, one should look at the subject’s stage of puberty, rather than the age. Further, instead of reporting the first encounter at the gender clinic, stage of puberty data should be analyzed based on the first documented instance of gender incongruence in the subject’s medical history. These data should be collected through a comprehensive chart review.
The results of this study have very limited applicability to practice outside the Amsterdam clinic.
The study acknowledges that its results are limited by having “originated from 1 center... [and] the results may be different for centers following a different treatment approach.” However, the authors do not adequately explain why this disclaimer is particularly important in this case. Prior to 2018, Amsterdam’s VUmc clinic was overseeing care for over 95% of gender dysphoria cases. However, starting about 2018 it began to redirect new patients to other clinics, and as of 2023 it is projected to serve less than 20% of youth cases. As 80% or more of Dutch gender-dysphoric youth are now treated at a dozen or more clinics, it is unclear whether the clinicians working in these other clinics will follow the same diagnostic and treatment approaches as practiced in the Amsterdam clinic.
The results of this study have very limited applicability to currently presenting cases.
The study’s data collection ended in 2018. Even for the patients that presented at the clinic in the last few years of the study, the effective follow up from treatment initiation is limited, and a number of those treated may not even be included in the analysis due to the exclusion for “ineligibility” described earlier. Since 2018, referrals of gender dysphoric youth have continued to surge in the Netherlands and worldwide, while the clinical presentations have continued to evolve, including the trend toward late-onset gender dysphoria, the preponderance of females, high rates of mental illness and neurocognitive comorbidities (e.g., ADHD, autism), and “nonbinary” presentations. At the same time, the Western world, and apparently the Dutch clinicians themselves, have moved away from the strict Dutch Protocol criteria that primarily reigned during much of the 20 years this study's data collection.
Thus, data gathered prior to 2018 already have limited applicability to the current clinical presentations. The significant departure from the original Dutch Protocol’s strict criteria, evident in the Dutch 2018 Somatic (medical) treatment guidelines, further limits the applicability of the findings obtained in prior years. (An upcoming update to the Dutch guidelines, expected in August 2023, may once again “reset” the clock if the criteria for treatment eligibility are changed once again.)
The study does not address trends in 18-25-year-old gender-dysphoric youth.
As the recent UK’s Cass review letter indicates, a substantial number of those presenting to adult gender clinics have just turned 18 and are under 25. The study’s Figure 6 also shows that a number of 17-year-olds “aged out” of the pediatric setting while undergoing assessments. Youth aged 18-25 are a highly vulnerable group of patients subject to similar epidemiologic trends as the adolescents. This group also is showing a rapidly rising incidence of gender dysphoria, is largely female, and has high rates of co-occurring mental health difficulties.
Future research should move beyond the somewhat arbitrary cut-off of age 18, analyzing the entire population of gender-dysphoric young people 25 and younger.
Final Thoughts
International readers asking key questions about the practice of youth gender transition are unlikely to find clear, reliable answers in this recent study from the Amsterdam gender clinic. While the referral trends through 2018 are likely accurate, the treatment trends reported by the study are hard to interpret, and some are misleading. The treatment trend calculations appear to include those not diagnosed with gender dysphoria in adolescence. They also appear to exclude at least some youth that were treated with puberty blockers, cross-sex-hormones, and surgery in the last few years of the study, when the practice began to deviate from the Dutch Protocol’s strict minimum age requirements.
Thus, this study is best understood as a time capsule of the “Dutch experience” from shortly after the inception of the practice of youth gender transition, through the first several years of the unprecedented surge in gender dysphoric youth. The study provides clear evidence of rapidly rising referrals in recent years, of the female preponderance, and of a high rate of initiation of medical transition in the older-presenting cohorts. The data also show extremely high rates of conversion from puberty blockers to cross-sex hormones.
The balance of the data presented in the study are subject to many irregularities in their definitions and calculations, which limit their utility. One of the study’s main limitations is that it appears to conflate two eras: the earlier time period when few (primarily male) pre-pubertal children presented to the Amsterdam clinic, and even fewer returned as adolescents to be treated with the original Dutch Protocol, and the later era of exploding numbers of female post-pubertal youth, who were treated using much less restrictive criteria. The fact that the study ended its data collection in 2018 poses additional challenges, as the populations of gender dysphoric youth have continued to grow and change in clinical presentations (including the rapidly growing numbers of youth who identify as nonbinary).
While the data presented in the study are often convoluted, the study’s title and abstract make a number of clear claims that can be easily misinterpreted as endorsement of current practices. However, these claims do not hold up to scrutiny when one reads beyond the abstract and has the skill to identify methodological problems. Few busy clinicians have the time or skill to engage in an in-depth critical appraisal of this and other studies, and thus, they rely on abstracts. In this sense, the study follows the unfortunate trend of many other recent studies in gender medicine, where there is a “marked asymmetry in outcomes reporting: findings of positive outcomes of medical interventions are trumpeted in abstracts, while their profound limitations remain... below the radar of busy clinicians.”
With 2-9% of secondary school and college-aged youth declaring a trans identity and many seeking evaluation and treatment, the question about how to best care for the skyrocketing numbers of such youth going forward is one of the most urgent dilemmas facing clinicians today. It is vitally important to design and conduct rigorous studies in order to begin to generate data that can reliably guide clinical decision-making.
Before endorsing continued use of puberty blockers, cross-sex hormones and surgery, the field of gender medicine must interrogate and determine why the number of gender-distressed youth is exploding—and why so many more are adolescent females. While the Dutch authors note an unprecedented rise in adolescent females, they opine that this is because “in most Western cultures, it is more widely accepted for AFAB [assigned females at birth] to come out as trans men...” This is an unlikely explanation, supported by a single reference to a 2013 paper that posits a range of theories for the increasing number of females, only one of which speculates that “masculine behavior is subject to less social sanction than feminine behavior.” Many would agree that there is a greater acceptance of “tomboys” compared to feminine boys in modern societies. However, this would suggest that girls with masculine preferences should find it easier to fit in as they are, while feminine boys might choose to transition more frequently in order to make their presentation more socially acceptable.
While the authors accept that their explanation for the sharp rise in adolescent girls presenting with gender dysphoria may be inadequate, they cite a lack of competing valid hypotheses. Yet the well-documented phenomenon of “peer contagion” spreading through social circles, with a clear preponderance of females, for a wide range of conditions from eating disorders to self-harm, is not mentioned as a possible mechanism. In the context of gender, the theory of peer contagion has been provisionally termed “ROGD” and has been endorsed by growing numbers of clinicians and detransitioners.
A 2022 paper from the Amsterdam clinic studying the same cohort concluded that “although there may be different developmental [paths] in adolescents that lead to seeking gender-affirming medical care, our data do not allow us to conclude whether or not this suggested ‘ROGD’ subtype exists.” However, the authors appear to be discouraging the hypothesis, based on their finding that “there was gender nonconformity in childhood in older presenters, although less extreme than in the younger presenting group, which speaks against this suggested subtype.” This is not a robust explanation. Besides the questionable selection of age 14 to separate youth into “old presenting” vs “early presenting” group—both an arbitrary cutoff, and one that misses the onset of puberty for most females—this question relies on the self-report by gender-dysphoric youth. However, many teens’ claims of enduring and extreme gender non-conformity and transgender identity from early childhood on are contradicted by parental reports.
As teens learn of the requirement of long-lasting gender dysphoria to be eligible for medical treatments, there may be an increase in instances of youth amending their prior histories to more readily gain access to puberty blockers, cross-sex hormones, and surgery. The Dutch team observed this tendency as early as 2005, reflecting on the possibility of both, “an (unconscious) exaggeration of history if current feelings are not clear-cut, or a conscious effort to mislead the clinician.” This phenomenon of knowing “exactly what to say” and importantly “what NOT to say” to facilitate access to “affirming” hormones has also been described by clinicians and parents.
Researchers would be well-advised to scrutinize medical records for a prior diagnosis of childhood gender dysphoria or to look for other credible, objective evidence of lifelong gender distress, rather than accepting patients’ accounts at face value. Thus, the 2022 study’s conclusion that “ROGD subtype likely does not exist,” made largely based on the youths’ self-report, is unreliable.
The scientific method requires progression from observations to hypothesis formation to hypothesis testing. Like all theories, the ROGD hypothesis must be subjected to rigorous testing, along with other competing hypotheses. However, when testing the ROGD hypothesis, it is important to test its actual key elements, rather than creating “straw-man” versions of what ROGD is in order to quickly refute it, as has been the case in many recent attempts to disparage or “disprove” it.
Contrary to the study’s assertions of having presented reliable long-term data that support ongoing medicalization, the growing voices of detransitioners (some of whom say they were treated at the Amsterdam clinic), and preliminary patient-level outcome data from the Dutch clinicians themselves, signal problems. Even patients who transitioned under the strict version of the Dutch Protocol appear to have substantial reproductive regret, body shame, and sexual dysfunction. These preliminary findings, presented by the Dutch clinicians at the WPATH Symposium in late 2022, serve as a potent reminder that puberty blockers, cross-sex-hormones and surgery remain experimental treatments, with an unknown risk-benefit ratio.
We hope that the upcoming studies from the Amsterdam clinic will analyze all the available data and address the following key questions:
How have the sex ratios of gender-dysphoric youth changed over the years?
What percent of cases presenting in recent years have early-onset vs pubertal-onset of gender dysphoria (validated by medical chart reviews rather than self-report)?
What percent of presenting cases are diagnosed as having gender dysphoria (for both types of presentations)?
What is the rate of co-occurring mental illness and neurocognitive disorders such as autism or ADHD?
How many of the diagnosed cases are deemed eligible to start medical treatment, and what are the eligibility criteria?
How many of those eligible to start medical treatments do so, and how many choose alternatives such as psychotherapy or supportive watchful waiting?
What are the rates of stopping treatment / detransitioning?
What are the short- and long-term psychological and physical health outcomes of those who medically transitioned? Long-term outcomes of 55 participants in the seminal 2014 study are of particular interest.
How do the outcomes of medical transition compare to the outcomes of those who chose alternative treatments such as psychotherapy, or chose no treatments?
Until there is reliable evidence that the benefits of youth gender transition outweigh the risks, it is critical to limit medical interventions to rigorous clinical research settings, while continuing to develop noninvasive approaches to help the rapidly growing number of gender-dysphoric youth worldwide.
Note: Among the many studies SEGM has analyzed, this has been one of the most challenging to decipher and analyze to date. We welcome dialogue and debate with the scientific community and the study authors about our interpretation of the trends reported.








Evidence that this is better than a placebo remains zero.
"The authors make several claims which, while technically accurate, may inadvertently be misleading."
There is nothing "inadevertent" about what these criminals are doing.